SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW
This site is intended for US residents.
SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

JIVI® VS ADYNOVATE® PK CROSSOVER STUDY
First read about the PK parameters of Jivi® from the PROTECT VIII trial, which will be helpful to understanding the data below from the crossover study examining the PK characteristics of Jivi® and Adynovate®.
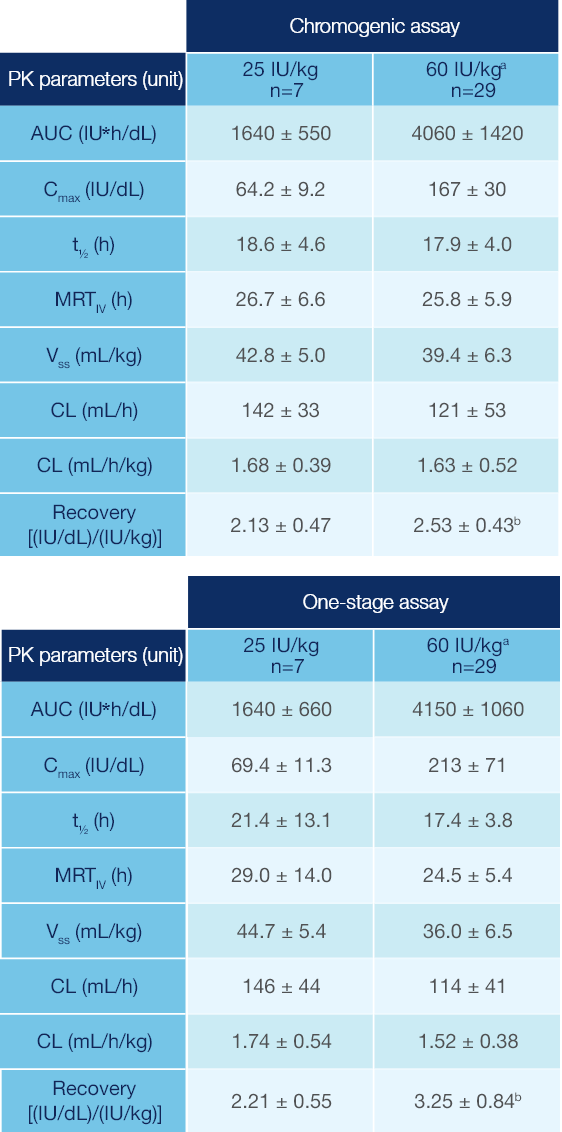
PK Parameters of Jivi® in Patients ≥12 Years of Age1
PK parameters of Jivi® in the PROTECT VIII trial (arithmetic mean ± SD)1
Measured following a single dose (25 IU/kg and 60 IU/kg)1
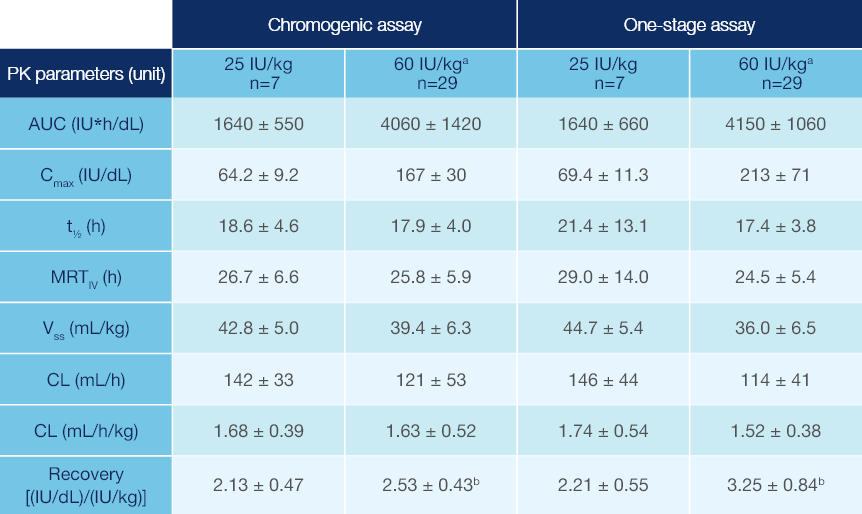
Combined data from Phase 1 and Phase 2/3 studies.
Recovery value could not be calculated for one subject.
PK: pharmacokinetic; SD: standard deviation; AUC: area under the curve; Cmax: maximum drug concentration in plasma after single dose;
t1/2: terminal half-life; MRTIV: mean residence time after an IV administration; VSS: apparent volume distribution at steady-state; CL: clearance.
Combined data from Phase 1 and Phase 2/3 studies.
Recovery value could not be calculated for one subject.
PK: pharmacokinetic; SD: standard deviation; AUC: area under the curve; Cmax: maximum drug concentration in plasma after single dose; t1/2: terminal half-life; MRTIV: mean residence time after an IV administration; VSS: apparent volume distribution at steady-state; CL: clearance.
Crossover study examining PK characteristics of Jivi® and Adynovate® (N=18)2*
The small size of the patient cohort could be a potential limitation of this study.2
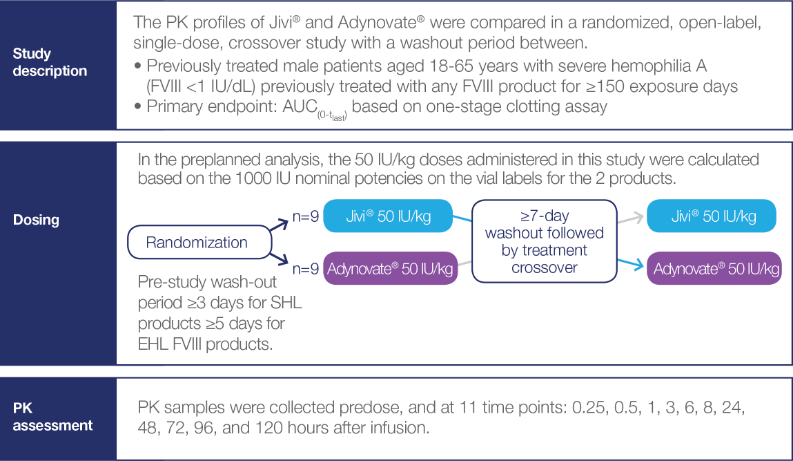
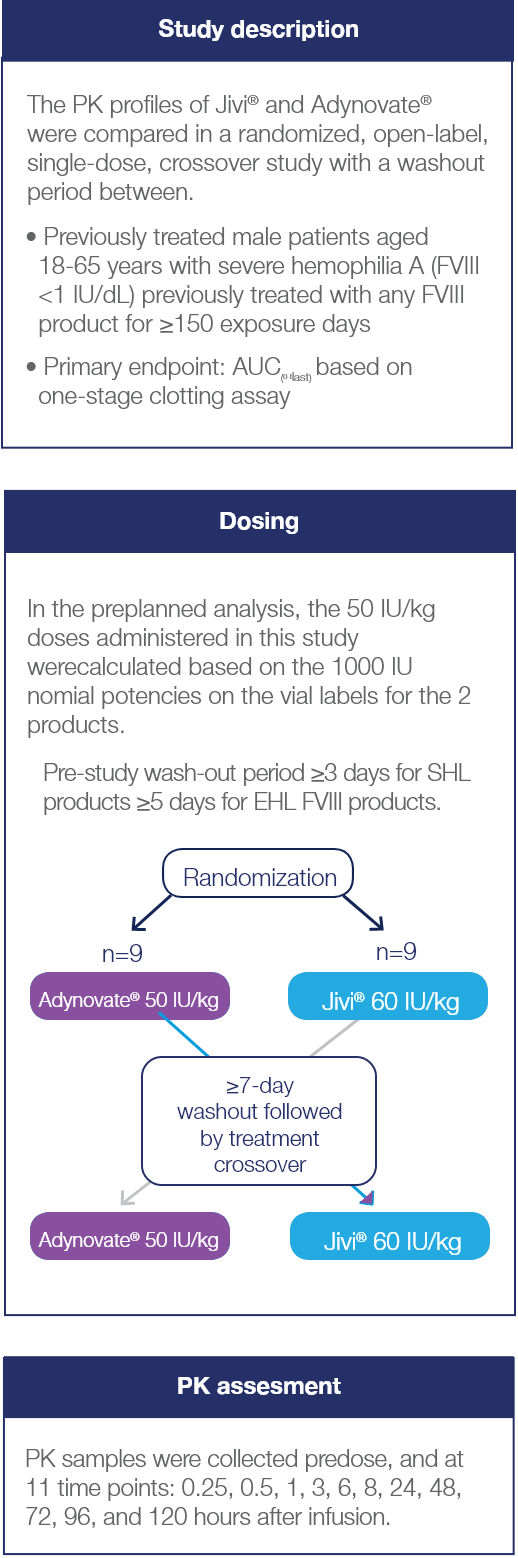
PK Analyses
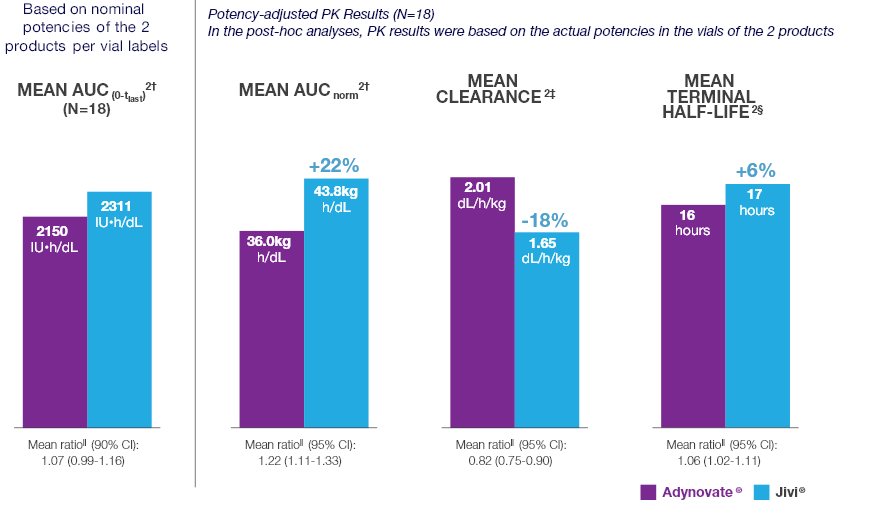
Potency-adjusted PK analyses of Jivi® and Adynovate® 2*
The preplanned analysis was followed by a post hoc analysis
- In the preplanned analysis, the 50 IU/kg doses administered in this study were calculated based on the 1000 IU nominal potencies provided on the vial labels of the 2 products
- In this study, the actual potencies were 1030 IU/vial for Jivi® and 1141 IU/vial for Adynovate®. This resulted in actual administered doses which were approximately:
- 3% higher than the planned 50 IU/kg dose for Jivi®
- 14.1% higher than the planned 50 IU/kg dose for Adynovate®
- A subsequent post hoc analysis of PK parameters was conducted using the actual potencies of the 2 products being compared
Comparative PK Results
Comparative PK results for Jivi® vs Adynovate®2
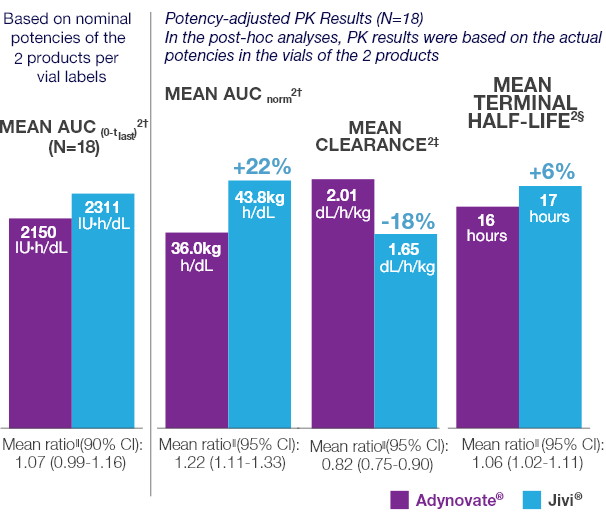
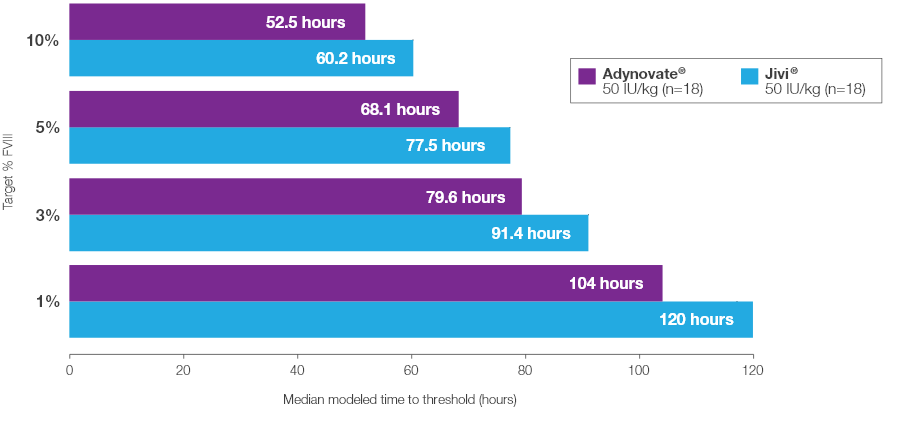
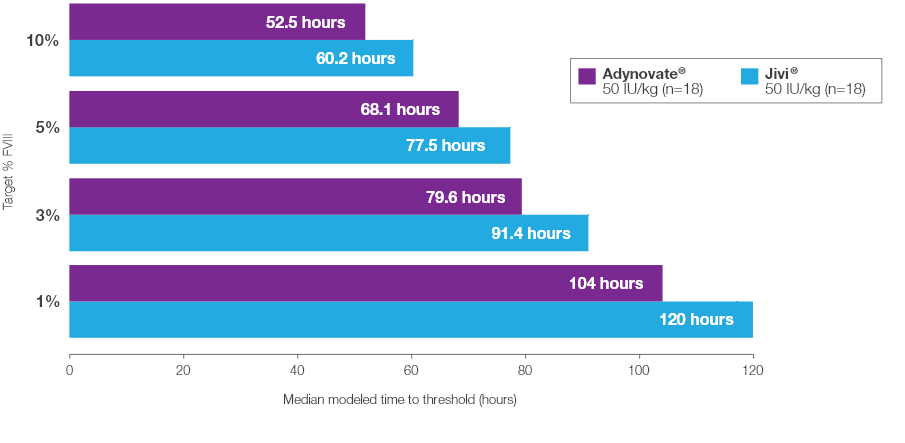
Time to Target Threshold Levels
Median time to target FVIII threshold levels with Jivi® vs Adynovate® 2
Estimated from a popular PK model (N=18) based on potency-adjusted results2*
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
References: 1. Jivi® Prescribing Information. Whippany, NJ: Bayer LLC; May 2025. 2. Solms A, Shah A, Bemtrop E, et al. Direct comparison of two extended half-life PEGylated recombinant FVIII products: a randomized, crossover pharmacokinetics study in patients with severe hemophilia A. Ann Hematol. Published online September 24, 2020. doi.10.1007/s00277-020-04280-3. 3. Anderson PL. The ABCs of pharmacokinetics. http://www.thebody.com/content/art875.html. Accessed April 2018. 4. Dhillon S, Gill K. Basic pharmacokinetics. In: Dhillon S, Kostrzewski A, eds. Clinical Pharmacokinetics. London, UK: Pharmaceutical Press; 2006. 5. Ratain MJ, Plunkett WK Jr. Principles of pharmacokinetics. In: Kufe DW, Pollock RE, Weichselbaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario: BC Decker; 2003.




