SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW
This site is intended for US residents.
SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

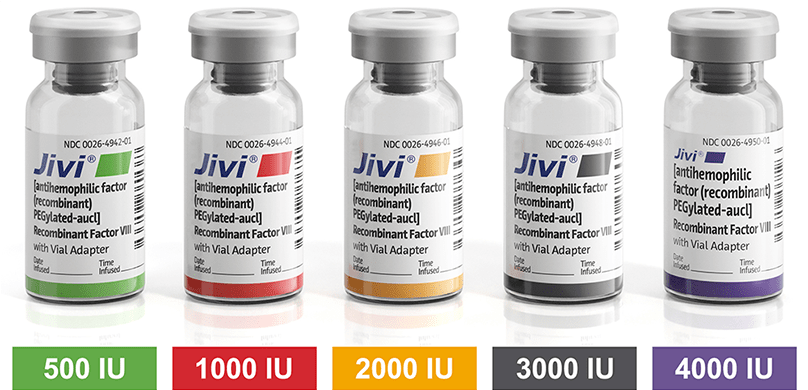
Jivi® is available in a range of vial sizes.1
500 IU Range
1000 IU Range
2000 IU Range
3000 IU Range
4000 IU Range (New!)
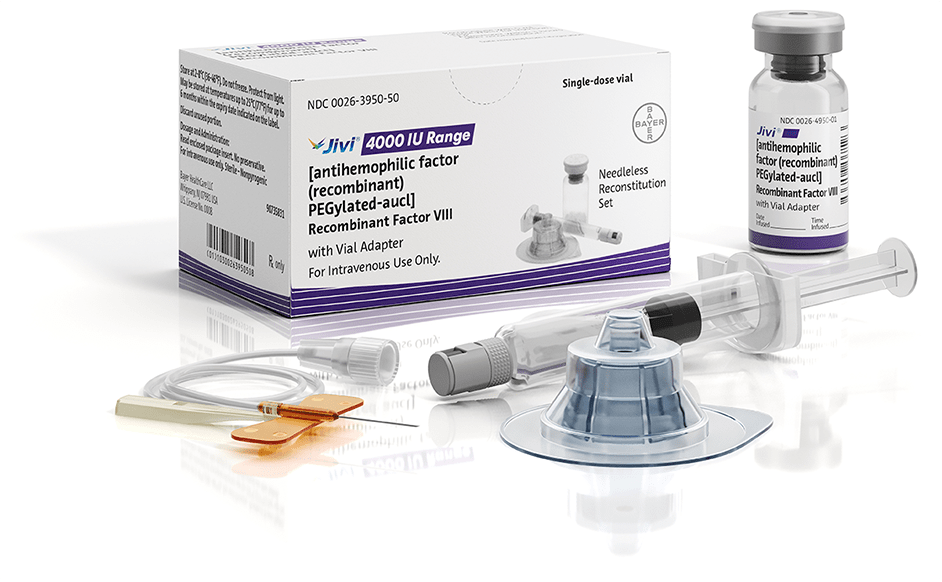
Jivi is available with the vial adapter
needleless reconstitution system, which contains1:
Vial adapter, with built-in 15-micrometer filter
2.5-mL diluent in a 5-mL syringe (500 IU, 1000 IU, 2000 IU and 3000 IU)
5.0-mL diluent in a 5-mL syringe (4000 IU only)
25-gauge butterfly needle

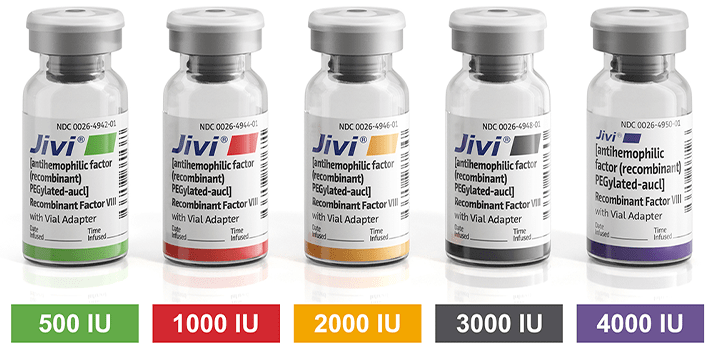
Jivi® is available in a range of vial sizes.1
500 IU Range
1000 IU Range
2000 IU Range
3000 IU Range
4000 IU Range (New!)
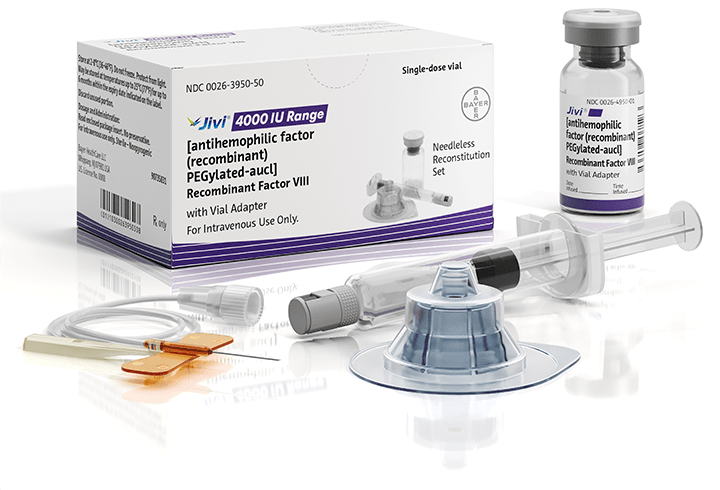
Jivi is available with the vial adapter needleless reconstitution system, which contains1:
Vial adapter, with built-in 15-micrometer filter
2.5-mL diluent in a 5-mL syringe (500 IU, 1000 IU, 2000 IU and 3000 IU)
5.0-mL diluent in a 5-mL syringe (4000 IU only)
25-gauge butterfly needle

Store Jivi at 36°F to 46°F for up to 24 months from the date of manufacture1:
Do not freeze1
Within this period, Jivi may be stored for a single period of up to 6 months at temperatures up to 77°F1
Record the starting date of room-temperature storage clearly on the unopened product carton1
Once stored at room temperature, do not return the product to the refrigerator1
The shelf life expires after storage at room temperature for 6 months, or after the expiration date on the product vial, whichever is earlier1
Store vials in their original carton and protect them from extreme exposure to light1
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
References: 1. Jivi® Prescribing Information. May 2025. Bayer. 2. Data on file. Tx Review 0918. Bayer; 2018. 3. Reding MT et al. J Thromb Haemost 2017;15:411-419. 4. Reding M, et al. Haemophilia. 2021; 10.1111/hae.14297.




