SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW
This site is intended for US residents.
SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

MONITORING FVIII ACTIVITY OF JIVI®
Monitoring Factor VIII Activity of Jivi® antihemophilic factor (recombinant) PEGylated-aucl
Jivi® Prescribing Information
Section 5.4 Monitoring Laboratory Tests1
If monitoring of Factor VIII activity is performed, use a validated chromogenic assay or a selected validated one-stage clotting assay.
Laboratories intending to measure the Factor VIII activity of Jivi® should check their procedures for accuracy. For Jivi®, select silica-based one-stage assays may underestimate the Factor VIII activity of Jivi® in plasma samples; some reagents, e.g., with kaolin-based activators, have the potential for overestimation.2 Therefore, the suitability of the assay must be ascertained. If a validated one-stage clotting or chromogenic assay is not available locally, then use of a reference laboratory is recommended.
Monitor for development of Factor VIII inhibitors. Perform a Bethesda inhibitor assay if expected Factor VIII plasma levels are not attained or if bleeding is not controlled with the expected dose of Jivi®. Use Bethesda Units (BU) to report inhibitor titers.
Jivi® FVIII activity can be monitored using the following chromogenic substrate assays and one-stage clotting assays.1,2,3* These chromogenic substrate assays can be used to monitor Jivi® alone or in presence of emiciumab-kxwh, as they contain Bovine reagents which are insensitive to emicizumab-kxwh.4
| Chromogenic substrate assays | One-stage clotting assays |
|---|---|
| Chromogenix Coamatic® (Instrumentation Laboratory) (Distributed by Diapharma)5 |
HemosIL® SynthASil (Instrumentation Laboratory) |
| Chromogenix Coatest® SP FVIII (Instrumentation Laboratory) (Distributed by Diapharma) |
Dade Actin® FSL (Siemens) |
| Pathromtin® SL (Siemens) |
Coamatic is a registered trademark of Instrumentation Laboratory S.P.A.; HemosIL is a registered trademark of Instrumentation Laboratory Company; Actin is a registered trademark of Siemens Healthcare Diagnostics Inc.; Pathromtin is a registered trademark of Siemens Healthcare Diagnostics Products GmbH. Coatest is a registered trademark of Instrumentation Laboratory Company.
*Based on results from either a field study or External Quality Control for Assays and Tests (ECAT) evaluations, these validated assays can be used to evaluate the activity of Jivi®.
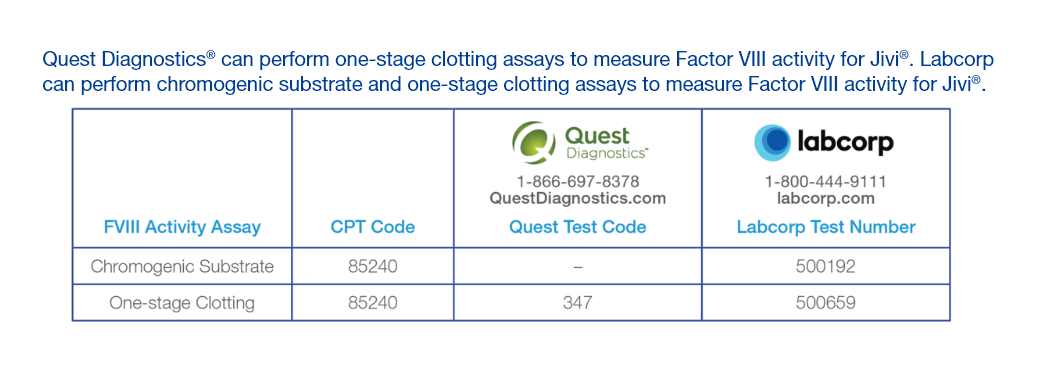
Quest Diagnostics is a registered trademark of Quest Diagnostics.
Labcorp is a registered trademark of Laboratory Corporation of America® Holdings.
Contact Access Services by Bayer™ to learn more about Jivi Reimbursement for Lab Testing
Contact Access Services by Bayer™ to learn more about Jivi Reimbursement for Lab Testing
1-800-288-8374
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
References: 1. Jivi® Prescribing Information. Whippany, NJ: Bayer LLC; May 2025. 2. Church N, Leong L, Katterle Y, et al. Factor VIII activity of BAY 94-9027 is accurately measured with most commonly used assays: results from an international laboratory study [published online July 8, 2018]. Haemophilia. doi:10.1111/hae.13564. 3. Data on File. Bayer Healthcare LLC, Whippany, NJ. 4. HemLibra® Prescribing Information. San Franciso, California: Genentech Inc.; 2021. 5. Chromogenix COAMATIC® VIII Package Insert. Chromogenix Instrumentation Laboratory Company; Bedford, MA. 6. Chromogenix COATEST® SP FVIII Package Insert. Chromogenix Instrumentation Laboratory Company; Bedford, MA.




