SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW
This site is intended for US residents.
SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

SELECTED IMPORTANT SAFETY INFORMATION: JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product. CONTINUE READING BELOW

Jivi — With Them as They Grow™
Proven Safety and Efficacy with Jivi: Clinical Study Data for Children Ages 7 to <12
*Please see full Jivi Prescribing Information for complete dosing information.
Study Design: PROTECT Kids and Alfa-PROTECT
PROTECT Kids
A multi-center, prospective, single-arm trial to evaluate the pharmacokinetics, safety and efficacy of Jivi for prophylaxis and treatment of bleeding in previously treated pediatric patients <12 years of age with severe hemophilia A (n = 73).1
Alfa-PROTECT
A multi-center, prospective, single-arm study to evaluate the safety of Jivi infusions for prophylaxis and treatment of bleeding in previously treated pediatric patients 7 to <12 years of age with severe hemophilia A (n=35).1
Pooled PROTECT Kids and Alfa-PROTECT
The PROTECT Kids and Alfa-PROTECT data were pooled as both studies included participants 7 to <12 years of age. The studies were designed to describe the safety and clinical efficacy of Jivi in previously treated participants 7 to <12 years of age with severe hemophilia A.1
Characteristics of Pooled Analysis Set1
Children PTPs (7 to <12 years of age)*
57*
ITT population for main efficacy analysis†
42 (prophylaxis twice weekly)‡
Treatment duration (main efficacy period)
26 weeks
The efficacy of prophylactic treatment was assessed in 57 patients 7 to <12 years of age across PROTECT Kids and Alfa-PROTECT
†ITT: Intention-to-treat
‡15 patients were on an extended interval regimen (every 5 days or every 7 days) in PROTECT Kids, so they are not included in the efficacy data
Safety and Tolerability: PROTECT Kids
and Alfa-PROTECT
A demonstrated safety profile in children 7 to <12 years of age
The most common adverse reactions (incidence ≥5%) in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough and abdominal pain.1
- There were no study drug-related serious adverse events2
- All drug-related events were mild or moderate2
- Drug-related AEs: 6.7% (n=4)2
- Zero FVIII inhibitors occurred2
One pediatric patient developed high titer neutralizing anti-PEG IgM antibodies (titer 1:64) after 2 exposure days associated with low post-infusion FVIII levels and loss of efficacy. Antibodies disappeared after discontinuation and patient restarted treatment with Jivi 2 months later.1
- Three pediatric patients developed low titer and transient anti-PEG IgM antibodies (highest titer 1:4) within the first 4 exposure days resulting in reduction of Jivi recovery (lowest recovery 0.8 kg/dl) in two patients and inconclusive data on recovery in one patient.1
Dosing: PROTECT Kids and Alfa-PROTECT
In the PROTECT Kids and Alfa-PROTECT studies, the median Jivi prophylaxis dose was 51.7 IU/kg, two times per week.1
Children (7 to <12 years of age)1* (N=42)
Dosing frequency: 2 times per week (25-60 IU/kg)
Median prophylaxis dose/infusion (range): 51.7 IU/kg (22-69 IU/kg)
Jivi approved dosing for children 7 to <12 years of age:
The recommended initial Jivi dosage regimen is 60 IU/kg twice weekly. The patient’s dose should be adjusted based on clinical response and/or recovery.1
Pooled patients from PROTECT Kids and Alfa-PROTECT 7 to <12 years of age with at least a 3-month treatment period, modified intention-to-treat (ITT) set
Effective Bleed Protection with Jivi: Annualized Bleed Rates (ABRs)*
In the PROTECT Kids and Alfa-PROTECT studies, ABRs for treated bleeds were assessed for Jivi in patients 7 to <12 years of age as a key secondary endpoint. The median ABR for total bleeds, joint bleeds, spontaneous bleeds and trauma bleeds was zero.2
Modified ITT population included patients with at least a 3-month treatment period
†Total bleeds include spontaneous bleeds, trauma bleeds and joint bleeds
‡Pooled patients from PROTECT Kids and Alfa-PROTECT 7 to <12 years of age, modified ITT set
Effective Bleed Protection with Jivi: Zero-Bleed Rates
Percentage of patients with zero bleeds during the entire study period of 26 weeks was 67%.1 Given variation in patients’ treatment start date and exact study phase duration, zero-bleed rate data broken out by bleed type are presented for the last 24 weeks of the 26-week study period.
In the PROTECT Kids and Alfa-PROTECT studies, majority of patients experienced zero treated bleeds, including spontaneous bleeds, joint bleeds, trauma bleeds and target joint bleeds.2
Total bleeds include spontaneous bleeds, trauma bleeds and joint bleeds
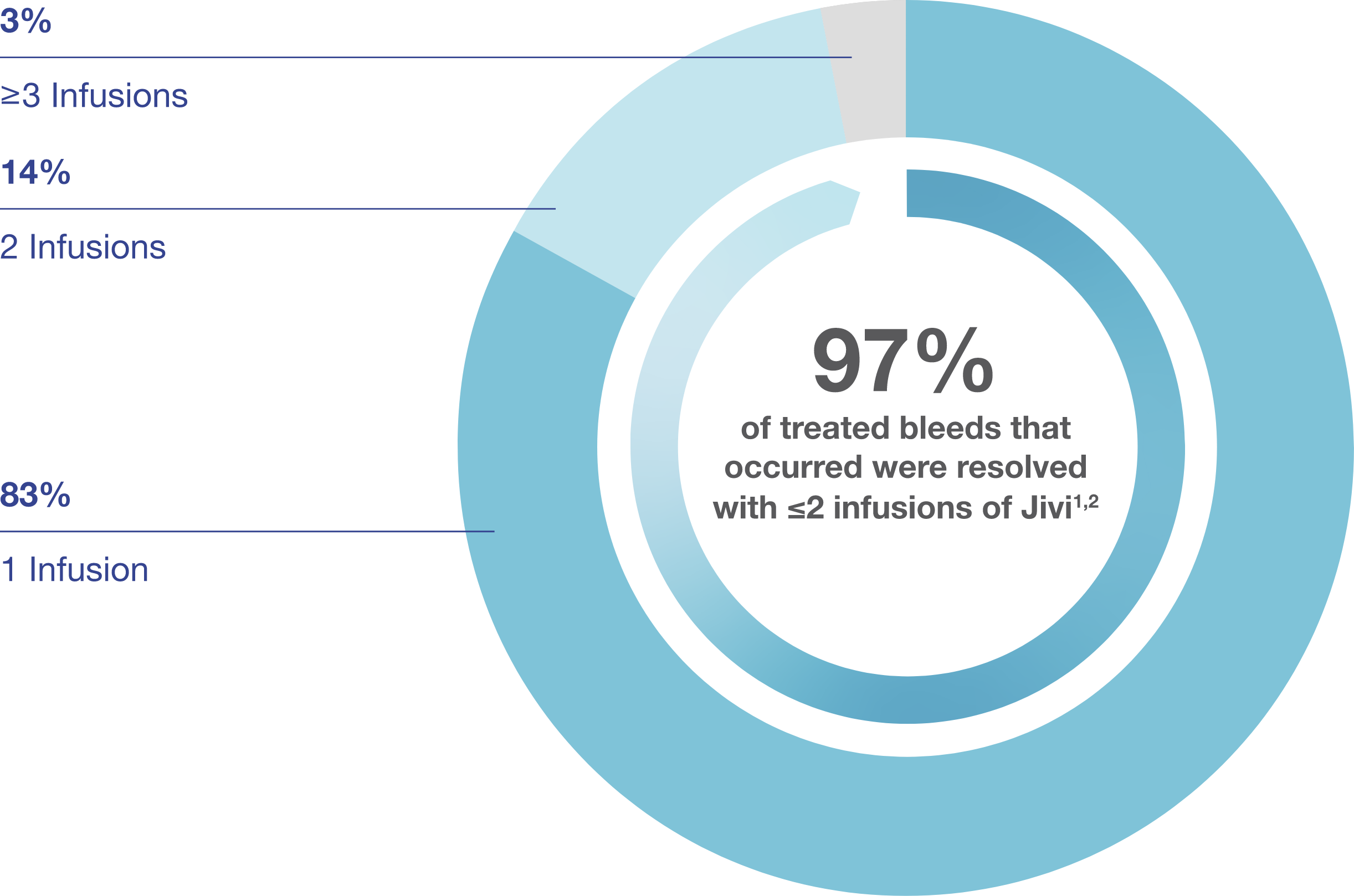
Effective Treatment of Bleeds with Jivi: Bleed Control
In the PROTECT Kids and Alfa-PROTECT studies, a total of 36 bleeding episodes were treated with Jivi in the 7 to <12 year-old population. 97% of bleeds were successfully treated with one or two infusions. The mean (SD) follow-up time between infusions was one (0.8) day.1,2
Pharmacokinetics (PK): PK Parameters of Jivi
Chromogenic Substrate Assay1 60 IU/kg
Results expressed as arithmetic mean ± SD
AUC: area under the curve
Cmax: maximum drug concentration in plasma after single dose
t½: terminal half-life
CL: clearance
MRTIV: mean residence time after an IV administration
VSS: apparent volume distribution at steady-state
n=131
†n=141
‡Data obtained from all patients’ recovery samples, excluding those with anti-drug antibodies, on a twice weekly regimen (n=42) with a range of 1.1-3.8 kg/dL1
Jivi — With Them as They Grow™: Dosing in
Adults and Children
Dosing for Children 7 to <12 Years of Age
Unique Step-Wise Dosing for Patients ≥12 Years of Age
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
INDICATION
JIVI® is a recombinant DNA-derived, Factor VIII concentrate indicated for use in previously treated adults and pediatric patients 7 years of age and older with hemophilia A (congenital Factor VIII deficiency) for:
On-demand treatment and control of bleeding episodes.
Perioperative management of bleeding.
Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitations of use
JIVI is not indicated for use in:
Children <7 years of age due to a greater risk for hypersensitivity reactions and/or loss of efficacy.
Previously untreated patients (PUPs).
Treatment of von Willebrand disease.
IMPORTANT SAFETY INFORMATION
JIVI is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, polyethylene glycol (PEG), mouse or hamster proteins, or other constituents of the product.
Hypersensitivity reactions, including severe allergic reactions, have occurred with JIVI. Monitor patients for hypersensitivity symptoms. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. If hypersensitivity reactions occur, immediately discontinue administration and initiate appropriate treatment.
JIVI may contain trace amounts of mouse and hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Hypersensitivity reactions may also be related to antibodies against polyethylene glycol (PEG).
Neutralizing antibody (inhibitor) formation has occurred following administration of JIVI. Carefully monitor patients for development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody).
An immune response associated with IgM anti-PEG antibodies, manifested as symptoms of acute hypersensitivity and/or loss of drug effect, has occurred with JIVI administration. In the clinical trials, the IgM anti-PEG antibodies disappeared within 4-6 weeks. No immunoglobulin class switching from IgM to IgG has been observed.
A low post-infusion Factor VIII level, in absence of detectable Factor VIII inhibitors, may be due to loss of treatment effect related to high titers of anti-PEG IgM antibodies. In these cases, discontinue JIVI and switch patients to a different anti-hemophilic product.
A reduced recovery of Factor VIII after start of JIVI treatment may be due to transient low titers of anti-PEG IgM antibodies. In these cases, increase the dose of JIVI until recovery of Factor VIII returns to expected levels.
The most common (incidence ≥5%) adverse reactions in clinical trials in previously treated patients (PTPs) ≥7 years of age were headache, fever, cough, and abdominal pain.
For additional important risk and use information, please see the full Prescribing Information.
You are encouraged to report side effects or quality complaints of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
References: 1. Jivi Prescribing Information. May 2025. Bayer. 2. Data on file. Whippany, NJ: Bayer; 2024. Clinical Study Report B003163.




